partner with clinvia
Clinvia CDM services
At Clinvia, we provide end-to-end services that empower faster decision-making, improve data quality, and simplify operations. We help our partners bring therapies to market with confidence and agility.
Clinical Data
Management Services

End-to-End Clinical Data Management
Clinvia manages clinical studies of all sizes, from Phase I–IV, as part of our FSP model or standalone projects. Whether for Pharma, Biotech, or Medical Devices, our clinical data managers are ready. We support Medidata RAVE, VEEVA, CLARIO, MEDDRIO, and other leading EDC systems.

EDC Designing and Setup
At Clinvia, our “Quality First” approach ensures attention to detail from study startup to closure. We understand the critical role of data management in meeting key milestones. Our skilled, high-performing project and functional teams are ready to deliver the clinical data management support you need.
EDC Designing and Setup
Data Quality & Regulatory Compliance
Data quality and regulatory compliance are critical to every trial. Our team performs rigorous checks from entry to database lock, ensuring audit readiness and transparency. We align with FDA, EMA, and ICH guidelines to manage risk, maintain documentation, and support successful, validated submissions.
Biostatistics and
Programming
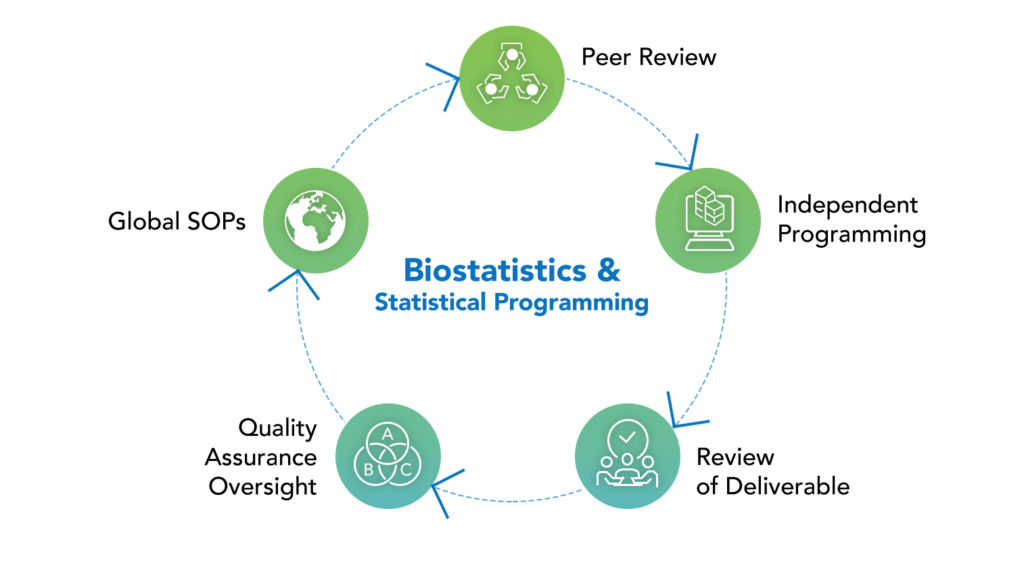

FSP & Standalone Biostatistics Support
Clinvia offers expert Biometrics services, supporting clinical studies from Phase I–IV through FSP models or standalone projects. Our experienced statisticians and programmers assist with all types of regulatory submissions and approvals.
Biometric Capabilities

Data Quality & Regulatory Compliance
Maintaining data quality and compliance is essential in every trial. Our Biometrics consultants apply rigorous checks from data entry to database lock. With a strong “Quality” focus, we ensure audit readiness and data transparency from the start.
Moreover…
As regulatory standards evolve, your data strategy must keep pace. Clinvia ensures alignment with FDA, EMA, and ICH guidelines, using practices that meet current expectations. We help manage risk, ensure proper documentation, and support successful submissions with complete, validated data.
